Risk Management
Risk Management Process, Risk Identification, Risk Analysis, Risk Evaluation, Risk Reduction (control the risk), Risk Monitoring, Risk Reviewing ……...
Risk Management is both prevalent and relevant in all industries. Whatever the Management System, whether well established and internationally recognized like:
• ISO 9001 Quality Management Systems
• ISO 13485 Medical Device Quality Management system
• Pharmaceutical Quality System (PQS)
• ISO 14001 Environmental
• ISO 45001 Occupational Health and Safety Management system
or
an independently implemented system, it is suffice to say that “Risk Management” is a critical component of that system.
The CORE risk management process can be defined as the “systematic application of management policies, procedures and practices to the activities of communicating, consulting, establishing the context, and identifying, analyzing, evaluating, treating, monitoring and reviewing risk (ISO 31000 Risk Management Principals and Guidelines).
Just like the guidance given in ISO 31000 (suitable for all industries) there are additional guidance documents supporting the implementation of effective risk management systems.
For example:
• ISO 14971 Medical Devices – Application of risk management to medical devices;
• ICH Q9 Pharmaceutical – Quality Risk Management;
• Health Service Executive (HSE National Health Authority) Integrated Risk Management Policy.
The Medical Device and Pharmaceutical industries impact all of our lives, (I think we can all agree) and are given a huge amount of trust by us when we avail of the services and products they provide. We all use medical devices and the vast majority of us use pharmaceuticals throughout our lives.
When comparing the Pharmaceutical Risk Management guidance (ICH Q9) to other guidance we find they are all based on having a documented Risk Management Process that includes Risk Identification, Risk Analysis/Assessment, Risk Evaluation, Risk Control / Reduction, Risk acceptance and Risk Review.
ICH Q9 ICH Q9 Pharmaceutical – Quality Risk Management
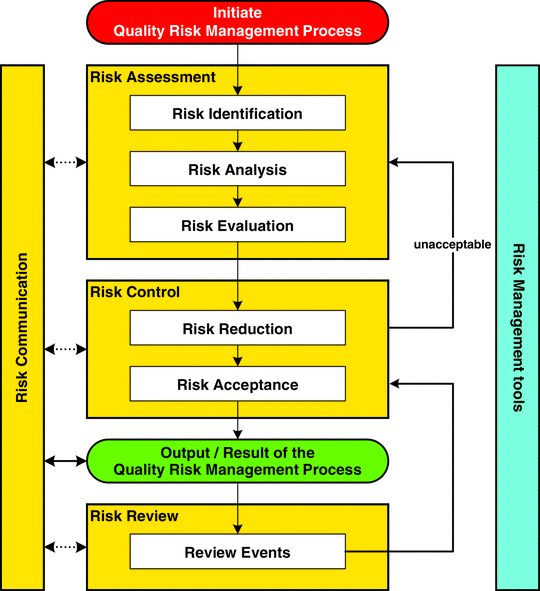
ICH Q9 specifically provides guidance on the principles and some of the tools of quality Risk Management that can enable more effective and consistent risk based decisions, both by regulators and industry, regarding the quality of drug substances and drug (medicinal) products across the product lifecycle.
Through my own research over the last 12 years I can safely say that no matter what industry you are in, a good understanding of what an effective Risk Management System entails is going to benefit both you and your organisation.
The Irish Quality Centre offer risk management training for general and specific industries.
Philip Byrne
Managing Director
Irish Quality Centre (IQC)
www.iqc.ie
philip@iqc.ie
#RiskManagement #RiskManagementProcess #RiskIdentification #RiskAnalysis #RiskEvaluation #RiskReduction #RiskMonitoring #Risk Reviewing





